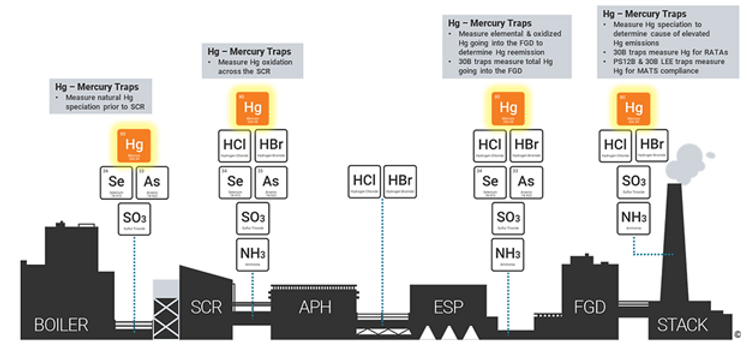
Many of our customers have found Mercury Speciation Sorbent Traps to be an excellent tool for evaluating the root cause of a mercury control problem. However, the traps can be VERY sensitive to heat. If the traps are being used without cooling to collect samples in an environment that is greater than 200°F, you may see a biased low oxidation rate because the oxidized Hg may breakthrough to the elemental Hg section, leading to an underestimation of your oxidation rate.
To avoid this, we recommend:
Using a cooling probe with a fine temperature control kit. This kit incorporates an in-line thermocouple to control probe temperature from the exhaust of each trap.
Performing a breakthrough study by altering sampling parameters (including flow rate, temperature, and duration) until you see movement from the oxidized Hg sections to the elemental Hg sections and then backing down the sampling parameters. We recommend doing this at each testing location before actual testing commences. For more details, please contact me!
Optimal sampling temperature for the cooling probe is typically 130°C (but this can vary from 100-150°C). If you sample at a temperature that’s too high, you will see movement of the oxidized Hg into the elemental Hg sections thus underestimating your oxidized Hg. If you sample at temperatures that are too low, you will form condensation inside of the trap and scrub elemental Hg thus overestimating your oxidation rate.
Optimal testing duration is 30 minutes (but ranges from 20-60 minutes depending on the Hg concentration.
Optimal sampling flow rate is 300cc/min (0.3lpm) but this also varies from 250cc/min – 500cc/min based on halogens and particulate as well as Hg concentration. If you sample at too low of a flow rate you may give time for elemental Hg to become oxidized or physically captured on the first plug and overestimating your oxidized Hg. If you sample at too high of a flow rate, then pressure will build and break the physical bonds that were formed by oxidized Hg and the sorbent meant to capture it. The oxidized Hg will then pass through those beds and be captured by the iodated carbon in the beds intended for elemental Hg thus underestimating your Hg oxidation.
You will need to control particulate entrainment because it often contains halogens that can scrub elemental mercury and bias your oxidation rate high. This can be done with static prefilters placed inside of each speciation trap, sintered titanium filters attached onto each sorbent trap with a Swagelok fitting, a probe shield placed onto the end of the probe, or an inertial attachment placed onto the end of the probe. The selection of these particulate reduction strategies is dependent on the flue gas characteristics at the testing location. We can help with figuring out which strategy to employ so don’t hesitate to contact me to discuss!
Analysis is also different for speciation sorbent traps so please contact me and I can send you SOPs for analysis as well as sampling.
Interested in learning even more? We are initiating our blog series again! Last year we published posts on factors that could be contributing to higher-than-expected Hg concentration levels. The first was High Hg upon Startup after a planned of unplanned outage and the second was using contaminated equipment. We’ve also shared a post about testing recommendations when you are seeing higher than expected Hg concentrations and if you are a lignite burning utility and worried about reducing Hg to meet potentially lower Hg limits. We’ll be posting even more articles over the next weeks, so stay tuned for updates.
While it all can sound a bit overwhelming at first, we’re here to help. We have a team of highly trained engineers and chemists on standby who would be happy to either walk you through the optimal sampling parameters or send a field engineer or chemist on-site to support you through your testing.
Next Up … how ammonia can affect SCR and Hg concentration levels.
Miss part of the series? Check out a few of our other blog posts on elevated Hg levels:
